Annonse
International Ovarian Tumor Analysis (IOTA) - methods for discriminating between benign and malignant adnexal masses
Publisert 20. mars 2018
Av: Lil Valentin. Senior professor in Obstetrics and Gynecology. Lund University, Sweden
Background
The International Ovarian Tumor Analysis (IOTA) collaboration was started in 1997 by myself, Dirk Timmerman, Tom Bourne, Ignace Vergote and William Collins. Today the IOTA collaboration has coworkers in more than 40 centers all over the world. The aim of the IOTA collaboration is to develop methods to correctly discriminate between benign and malignant adnexal masses and then to prospectively validate these methods. It has been shown in several studies that the best ultrasound method for discriminating between benign and malignant adnexal masses is subjective evaluation of the ultrasound image, i.e. pattern recognition, by a very experienced ultrasound examiner (1-3). However, not all who use gynecological ultrasound in their daily clinical practice are very experienced. Therefore, we, i.e. the five founders of the IOTA collaboration, wanted to develop methods that could help less experienced ultrasound examiners to correctly discriminate between benign and malignant adnexal masses. However, before embarking on any study to develop such methods we needed to agree on a standardized examination technique and measurement technique and on a common terminology to be used when describing ultrasound images adnexal masses. We came to a consensus and published our consensus statement in the year of 2000 (4). The IOTA examination technique, measurement technique and terminology are used in all IOTA studies and are now being introduced into clinical practice all over the world.
The IOTA methods
The IOTA group has developed five methods for discrimination between benign and malignant adnexal masses: Logistic regression model 1 (LR1) (5), Logistic regression model 2 (LR2) (5), the Assessment of Different NEoplasias in the adneXa (ADNEX) model (6), the Simple Rules (7) and the Simple Rules risk calculation model (8). All have been prospectively validated on more than 1000 masses and have been found to have excellent ability to discriminate between benign and malignant adnexal masses (9-30) and to be superior to the Risk of Malignancy Index (RMI) and to the Risk of Ovarian Malignancy Algorithm (ROMA) (11, 13, 19, 31-33). Moreover, the IOTA methods, especially ADNEX are clinically more useful than RMI and ROMA to select patients with adnexal masses for specialized oncology care (34).
LR1 and LR2
LR1 includes three clinical and nine ultrasounds variables, LR2 includes one clinical variable (the patient´s age) and five ultrasound variables. The variables are entered into a mathematical formula that calculates the risk of malignancy. A risk above 10% classifies the mass as malignant. An “app” for calculation of the risk of malignancy using LR1 and LR2 can be downloaded for free from App-store or Google play, or from the IOTA website ( www. iotagroup.org ).
ADNEX
The ADNEX model does not only classify a mass as benign or malignant but calculates the likelihood that a mass is benign, borderline malignant, stage 1 primary invasive ovarian cancer, stage 2-4 primary invasive ovarian cancer, or a metastasis in the ovary from another primary tumor. The ADNEX model includes three clinical variables and six ultrasound variables. One of the three clinical variables is serum CA125. CA125 is not needed for discrimination between benign and malignant masses, but adding CA125 to the ADNEX model improves the ability to correctly discriminate between the four types of malignancy. The variables in the ADNEX model are simple and robust: patient´s age, type of center in which the ultrasound examination was performed (oncologic referral center or other), largest diameter of the mass (mm), largest diameter of the largest solid component (mm), number of cyst locules > 10 (yes or no), number of papillary projections (0, 1, 2, 3, 4 or more), shadowing (yes or no), ascites (yes or no). The variables are entered into a mathematical formula that calculates the likelihood of each of the five types of tumor. We have shown that the calculated likelihood of a particular type of tumor agrees extremely well with the true prevalence of that type of tumor (6). The ADNEX model is available for free as a web application on the IOTA website. The ADNEX “app” can be bought on Appstore or Google Play. The App presents the baseline likelihood of the five types of tumor as well as the likelihood after the ADNEX app has been applied, so that the change in likelihood after ADNEX model has been applied can be calculated (Figure 1).

Figure 1. Output of the ADNEX model as shown when using the “app”.
IOTA Simple Rules
The IOTA Simple Rules have become very popular because they are very easy to use. They do not require access to a computer. Using the IOTA Simple Rules there are five malignant ultrasound features and five benign ultrasound features. The ten features are shown in Figure 2. If only malignant features are present, the mass is classified as malignant. If only benign features are present, the mass is classified as benign. If both benign and malignant features are present, or if none of the ten features is present, the mass cannot be classified using the Simple Rules. On prospective validation between 77% and 96% of all tumors were classifiable using the Simple Rules, and in most studies slightly less than 80% of the tumors could be classified as benign or malignant (10,11, 14-18, 25-29). There are different possibilities to manage masses unclassifiable by the Simple Rules: 1) one can classify all the inconclusive cases as malignant, in which case the of misclassifying a malignant mass as benign is extremely low, but many benign masses will be misclassified as malignant 2) one can refer the patient to an expert in gynecological ultrasound, who will then use pattern recognition to classify the mass, or 3) one can apply one of the IOTA mathematical models, e.g. the ADNEX model, or the Simple Rules Risk Calculation model. The Simple Rules Risk Calculation model uses the ten ultrasound features in the Simple Rules plus the type of center in which the patient was examined with ultrasound (oncology referral center or other) to calculate the risk of malignancy (8). This requires access to a computer, but one can also read off the risk of malignancy in a table (8).
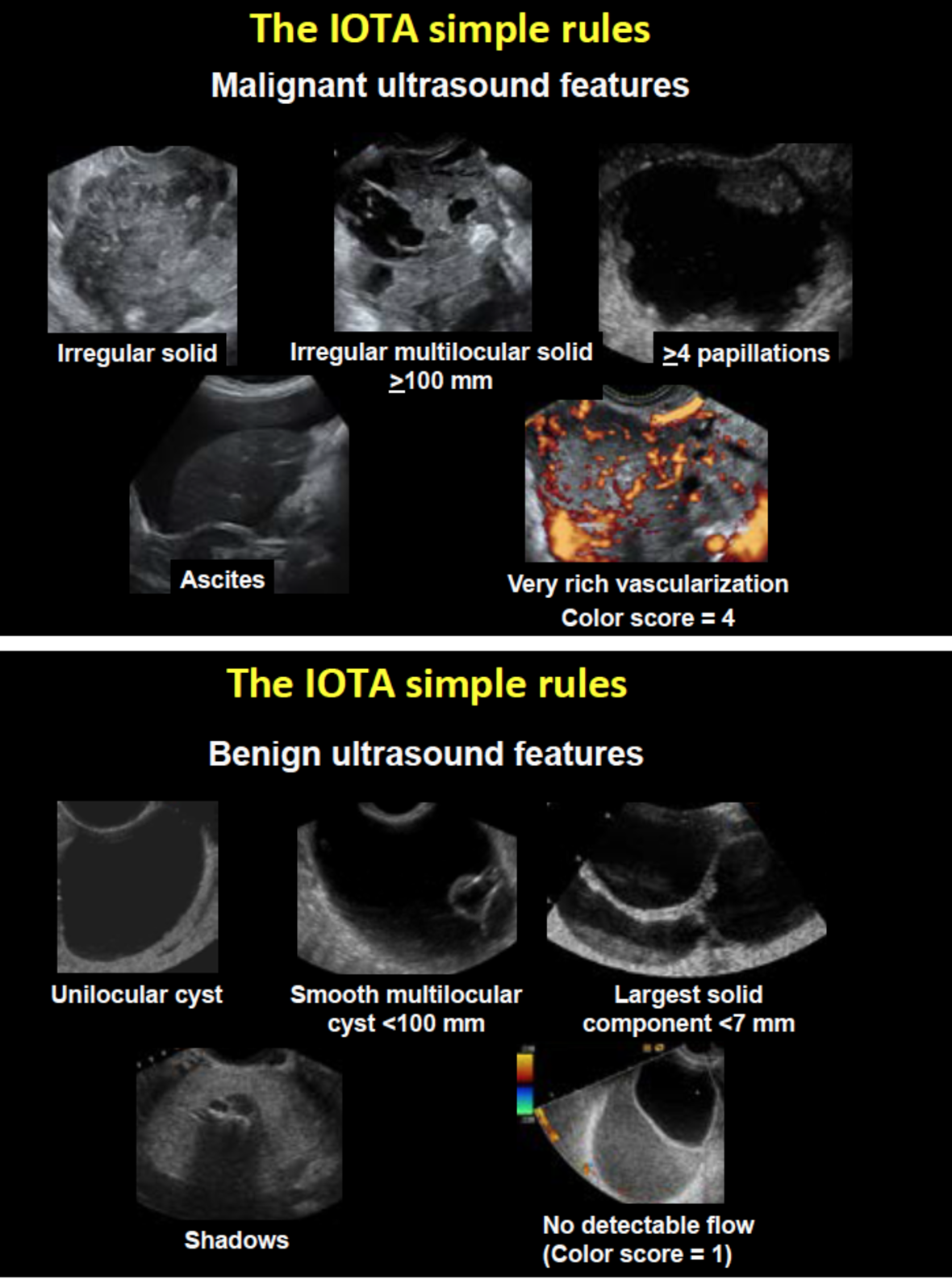
Figure 2. The malignant ultrasound features are shown in a) The benign ultrasound features are shown in b). If only malignant features are present, the mass is classified as malignant. If only benign features are present, the mass is classified as benign. If both benign and malignant features are present, or if none of the ten features is present, the mass cannot be classified using the Simple Rules.

Figure 3. A “traffic light system” showing an example on how one can use the ADNEX model in clinical practise. Depending on local conditions (available health care resources, local referral patterns and guidelines, and the level of oncological competence in non-oncology centers) other risk cutoffs for classifying patients as high risk, intermediate risk or low risk of having an adnexal malignancy may be suitable.
Which IOTA method to use?
One can choose whichever IOTA method one prefers personally, but one should stick to that method. If one uses the Simple Rules, one also needs choose which strategy to apply on the masses that cannot be classified using the Simple Rules (see above). The strategy that the IOTA steering committee currently wants to promote is the following: Classify all unilocular cysts with a largest diameter < 10 cm as benign. Virtually all (99-100%) of such mass are benign (35). Then apply the ADNEX model on the remaining masses. The ADNEX model classifies the mass as benign or malignant, and if malignant it provides information on the most likely type of malignancy. If one uses a risk of malignancy >10% to classify a mass as malignant, the risk of misclassifying a malignant mass as benign with the ADNEX model is minimal.
The outcome of the ADNEX-model can be used to help patient counselling and management. For example, if the risk of malignancy is >20% it might be advisable to refer the patient to a referral center for gynecological oncology (high risk group). If the risk of malignancy is < 1% (low risk group), the patient probably does not need surgery (unless she has symptoms related to the mass). If the risk is 1% to 20% (intermediate risk group), surgery might be needed, but not necessarily in a referral center for gynecological oncology (Figure 2). This is just an example. Depending on local conditions (available health care resources, e.g. restricted access to gynecological oncological surgical expertise, local referral patterns and guidelines, and the level of oncological competence in non-oncology centers) other risk cutoffs for referring patients to an oncology center or for managing them expectantly may be suitable. Practical tips on how to use and interpret the results of the ADNEX model in clinical practice can be found in reference 36.
IOTA certification
The IOTA methods work only if one uses the IOTA definitions of the variables in the models and the Simple Rules. For example, for the Simple Rules to work, one must know and use the IOTA definitions of unilocular cyst, multilocular cyst, solid tumor, multilocular solid tumor, solid component, papillary projection, ascites, and shadowing. One must know how to perform a gynecological Doppler ultrasound examination, i.e. how to adjust color Doppler settings to optimize detection of slow velocity blood flow, and one must use the IOTA measurement technique. In other words, one needs to be IOTA certified.
On the IOTA website, there are lectures available for free. One of these lectures (30 min) goes through the definitions of all IOTA variables (the definitions are also presented in the IOTA consensus statement (4)). There are different possibilities to take the IOTA certification test and become IOTA certified: attend an IOTA workshop, attend an IOTA Congress, or attend ultrasound courses that incorporate IOTA certification. Information about workshops, congresses and courses can be found on the IOTA website. Customized IOTA workshops including the certification test can be provided locally after contacting one of the members of the IOTA steering committee. IOTA lectures and training possibilities are also available on www.iota.education
Summary
- On prospective validation all IOTA methods have been found to perform well for discrimination between benign and malignant tumors.
- The IOTA methods are superior to RMI and ROMA.
- The IOTA methods, especially ADNEX, are clinically more useful than RMI and ROMA to select patients with adnexal masses for specialized oncology care.
- IOTA software is available for free on the IOTA website.
- “Apps” for IOTA LR1, LR2 and the Simple Rules are available for free on Google Play and in App-store.
- The IOTA methods work only if one uses the IOTA definitions of the variables in the models.
- Lectures on the IOTA methods are available for free on the IOTA website and on www.iota.education.
- IOTA workshops, IOTA courses and IOTA Congresses offering the IOTA test to become IOTA certified are announced on the IOTA website.
- More information about IOTA can be found on the IOTA website: www.iotagroup.org.
- IOTA is also on facebook.
Refererences
- Valentin L. Pattern recognition of pelvic masses by gray-scale ultrasound imaging: the contribution of Doppler ultrasound. Ultrasound Obstet Gynecol 1999; 14 : 338–347.
- Valentin L, Hagen B, Tingulstad S, Eik-Nes S. Comparison of ‘pattern recognition’ and logistic regression models for discrimination between benign and malignant pelvic masses: a prospective cross validation. Ultrasound Obstet Gynecol 2001; 18 : 357-365.
- Timmerman D. The use of mathematical models to evaluate pelvic masses; can they beat an expert operator? Best Pract Res Clin Obstet Gynaecol 2004; 18 : 91-104.
- Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H, Vergote I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol 2000; 16: 500–505
- Timmerman D, Testa AC, Bourne T, Ferrazzi E, Ameye L, Konstantinovic ML, Valentin L. Logistic regression model to distinguish between the benign and malignant adnexal mass before surgery: a multicenter study by the International Ovarian Tumor Analysis Group. J Clin Oncol 2005; 23: 8794–8801.
- Van Calster B, Van Hoorde K, Valentin L, Testa AC, Fischerova D, Van Holsbeke C, Savelli L, Franchi D, Epstein E, Kaijser J, Van Belle V, Czekierdowski A,Guerriero S, Fruscio R, Lanzani C, Scala F, Bourne T, Timmerman D; International Ovarian Tumour Analysis Group. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014 Oct 15;349:g5920.
- Timmerman D, Testa AC, Bourne T, Ameye L, Jurkovic D, Van Holsbeke C, PaladiniD, Van Calster B, Vergote I, Van Huffel S, Valentin L. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound in Obstetrics and Gynecology. 2008;31: 681-690.
- Timmerman D, Van Calster B, Testa A, Savelli L, Fischerova D, Froyman W,Wynants L, Van Holsbeke C, Epstein E, Franchi D, Kaijser J, Czekierdowski A,Guerriero S, Fruscio R, Leone FP, Rossi A, Landolfo C, Vergote I, Bourne T, Valentin L. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am J Obstet Gynecol. 2016;214:424-37.
- Timmerman D, Van Calster B, Testa AC, Guerriero S, Fischerova D, Lissoni AA, Van Holsbeke C, Fruscio R, Czekierdowski A, Jurkovic D, Savelli L, Vergote I, Bourne T, Van Huffel S, Valentin L. Ovarian cancer prediction in adnexal masses using ultrasound-based logistic regression models: a temporal and external validation study by the IOTA group. Ultrasound Obstet Gynecol. 2010;36:226-34.
- Timmerman D, Ameye L, Fischerova D, Epstein E, Melis GB, Guerriero S, Van Holsbeke C, Savelli L, Fruscio R, Lissoni AA, Testa AC, Veldman J, Vergote I, Van Huffel S, Bourne T, Valentin L. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010 Dec 14;341:c6839.
- Testa A, Kaijser J, Wynants L, Fischerova D, Van Holsbeke C, Franchi D, Savelli L, Epstein E, Czekierdowski A, Guerriero S, Fruscio R, Leone FP, Vergote I, Bourne T, Valentin L, Van Calster B, Timmerman D. Strategies to diagnose ovarian cancer: new evidence from phase 3 of the multicentre international IOTA study. Br J Cancer. 2014;111:680-8.
- Nunes N, Ambler G, Hoo WL, Naftalin J, Foo X, Widschwendter M, Jurkovic D. A prospective validation of the IOTA logistic regression models (LR1 and LR2) in comparison to subjective pattern recognition for the diagnosis of ovarian cancer. Int J Gynecol Cancer. 2013;23:1583-9.
- Sayasneh A, Wynants L, Preisler J, Kaijser J, Johnson S, Stalder C, Husicka R, Abdallah Y, Raslan F, Drought A, Smith AA, Ghaem-Maghami S, Epstein E, Van Calster B, Timmerman D, Bourne T. Multicentre external validation of IOTA prediction models and RMI by operators with varied training. Br J Cancer. 2013;108:2448-54.
- Sayasneh A, Kaijser J, Preisler J, Johnson S, Stalder C, Husicka R, Guha S, Naji O, Abdallah Y, Raslan F, Drought A, Smith AA, Fotopoulou C, Ghaem-Maghami S, Van Calster B, Timmerman D, Bourne T. A multicenter prospective external validation of the diagnostic performance of IOTA simple descriptors and rules to characterize ovarian masses. Gynecol Oncol. 2013;130:140-6.
- Alcázar JL, Pascual MÁ, Olartecoechea B, et al. IOTA simple rules for discriminating between benign and malignant adnexal masses: Prospective external validation. Ultrasound Obstet Gynecol. 2013;42:467-471.
- Tantipalakorn C, Wanapirak C, Khunamornpong S, Sukpan K, Tongsong T. IOTA simple rules in differentiating between benign and malignant ovarian tumors. Asian Pac J Cancer Prev. 2014;15:5123-6.
- Hartman CA, Juliato CRT, Sarian LO, et al. Ultrasound criteria and CA 125 as predictive variables of ovarian cancer in women with adnexal tumors. Ultrasound Obstet Gynecol. 2012;40:360-366.
- Fathallah K, Huchon C, Bats AS, et al. External validation of simple ultrasound rules of Timmerman on 122 ovarian tumors. Gynecol Obstet Fertil. 2011;39:477-481. Van Holsbeke C, Van Calster B, Bourne T, Ajossa S, Testa AC, Guerriero S,Fruscio R, Lissoni AA, Czekierdowski A, Savelli L, Van Huffel S, Valentin L, Timmerman D. External validation of diagnostic models to estimate the risk of malignancy in adnexal masses. Clin Cancer Res. 2012;18:815-25.
- Meys EM, Jeelof LS, Achten NM, Slangen BF, Lambrechts S, Kruitwa-gen RF, et al. Estimating the risk of malignancy in adnexal masses: external validation of the ADNEX model and comparison with other frequently used ultrasound methods. Ultrasound Obstet Gynecol 2017;49:784–92.
- Szubert S, Wojtowicz A, Moszynski R, Zywica P, Dyczkowski K, Stachowiak A, et al. External validation of the IOTA ADNEX model performed by two independent gynecologic centers. Gynecol Oncol 2016;142:490–5.
- Araujo KG, Jales RM, Pereira PN, Yoshida A, de Angelo Andrade L, SarianL O, et al. Performance of the IOTA ADNEX model in the preoperative discrimination of adnexal masses in a gynecologic oncology center. Ultra-sound Obstet Gynecol 2017;49:778–83.
- Epstein E, Van Calster B, Timmerman D, Nikman S. Subjective ultrasound assessment, the ADNEX model and ultrasound-guided tru-cut biopsy to differentiate disseminated primary ovarian cancer from metastatic non-ovarian cancer. Ultrasound Obstet Gynecol 2016;47:110–6.
- Sayasneh A, Ferrara L, De Cock B, Saso S, Al-Memar M, Johnson S, et al .Evaluating the risk of ovarian cancer before surgery using the ADNEX model: a multicentre external validation study. Br J Cancer 2016;115:542–8.
- Tinnangwattana D, Vichak-Ururote L, Tontivuthikul P, Charoenratana C, Lerthiranwong T, Tongsong T. IOTA Simple Rules in Differentiating between Benign and Malignant Adnexal Masses by Non-expert Examiners. Asian Pac J Cancer Prev. 2015;16:3835-8.
- Nunes N, Ambler G, Foo X, Naftalin J, Widschwendter M, Jurkovic D. Use of IOTA simple rules for diagnosis of ovarian cancer: meta-analysis. Ultrasound Obstet Gynecol. 2014;44:503-14.
- Tongsong T, Tinnangwattana D, Vichak-Ururote L, Tontivuthikul P, Charoenratana C, Lerthiranwong T. Comparison of Effectiveness in Differentiating Benign from Malignant Ovarian Masses between IOTA Simple Rules and Subjective Sonographic Assessment. Asian Pac J Cancer Prev. 2016;17:4377-4380.
- Knafel A, Banas T, Nocun A, Wiechec M, Jach R, Ludwin A, Kabzinska-Turek M, Pietrus M, Pitynski K. The Prospective External Validation of International Ovarian Tumor Analysis (IOTA) Simple Rules in the Hands of Level I and II Examiners. Ultraschall Med. 2016;37:516-523.
- Ruiz de Gauna B, Rodriguez D, Olartecoechea B, Aubá M, Jurado M, Gómez Roig MD, Alcázar JL. Diagnostic performance of IOTA simple rules for adnexal masses classification: a comparison between two centers with different ovarian cancer prevalence. Eur J Obstet Gynecol Reprod Biol. 2015;191:10-4.
- Koneczny J, Czekierdowski A, Florczak M, Poziemski P, Stachowicz N, BorowskiD. The use of sonographic subjective tumor assessment, IOTA logistic regression model 1, IOTA Simple Rules and GI-RADS system in the preoperative prediction of malignancy in women with adnexal masses. Ginekol Pol. 2017;88:647-653.
- Kaijser J, Van Gorp T, Van Hoorde K, Van Holsbeke C, Sayasneh A, Vergote I, Bourne T, Timmerman D, Van Calster B. A comparison between an ultrasound based prediction model (LR2) and the risk of ovarian malignancy algorithm (ROMA) toassess the risk of malignancy in women with an adnexal mass. Gynecol Oncol. 2013;129:377-83.
- Meta-analys Kaijser J, Sayasneh A, Van Hoorde K, Ghaem-Maghami S, Bourne T, Timmerman D, Van Calster B. Presurgical diagnosis of adnexal tumours using mathematical models and scoring systems: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:449-62.
- Meys EM, Kaijser J, Kruitwagen RF, Slangen BF, Van Calster B, Aertgeerts B, Verbakel JY, Timmerman D, Van Gorp T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur J Cancer. 2016;58:17-29.
- Wynants L, Timmerman D, Verbakel JY, Testa A, Savelli L, Fischerova D, Franchi D, Van Holsbeke C, Epstein E, Froyman W, Guerriero S, Rossi A, Fruscio R, Leone FP, Bourne T, Valentin L, Van Calster B. Clinical Utility of Risk Models to Refer Patients with Adnexal Masses to Specialized Oncology Care: Multicenter External Validation Using Decision Curve Analysis. Clin Cancer Res. 2017;23:5082-5090.
- Ameye L, Timmerman D, Valentin L, Paladini D, Jingzhang, Van Holsbeke C,Lissoni AA, Savelli L, Veldman J, Testa AC, Amant F, Van Huffel S, Bourne T. Clinically oriented threestep strategy to the assessment of adnexal pathology. Ultrasound Obstet Gynecol. 2012;40:582-91.
- Van Calster B, Van Hoorde K, Froyman W, Kaijser J, Wynants L, Landolfo C, Anthoulakis C, Vergote I, Bourne T, Timmerman D. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis Obgyn. 2015;7:32-41.